I good design practice for medical devices and equipment requirements capture written by. Design for validation article in journal of medical engineering technology 24253 62 march 2000 with 133 reads.
 Top 8 Healthcare Predictions For 2019
Top 8 Healthcare Predictions For 2019
A good time frame for a review is 60 90 minutes.
(PDF) Good Design Practice For Medical Devices And Equipment A Framework Download. Sandra shefelbine john clarkson roy farmer engineering design centre cambridge university and stephen eason cambridge consultants ltd. These lay the foundation for the rest of the design. The regulation provides a framework to implement the design control to a wide variety of devices.
Medical device design and development is a complex process rife with regulations specifications application requirements and end user needs all of which must be balanced and adhered to for a successful product. The good news is that with a few open source tools those sops can be put into practice and help you comply with regulations and create better products. If your device lacks usability market share will suffer but if your device doesnt meet regulatory guidelines it wont make it to.
Any shorter you wont get through everything that needs to be discussed. Best practices for design and development of software medical devices the bad news is that nobody including the author remembers exactly what those procedures say. Good design practice for medical devices and equipment part ii.
Keep your design reviews timely. Karen alexanderkaren alexander john clarksonjohn clarkson engineering design centre university of cambridge engineering design centre university of cambridge. Additionally fda incorporates current good manufacturing practice cgmp requirements into the quality system regulation with an aim to follow good quality practices for medical devices design.
This covers one of the key activities in the design process the systematic collection of design requirements. Any longer youll lose the attention of your audience. Good design practice for medical devices and equipment requirements capture is the first workbook in this series.
The second workbook in this series good design practice for medical devices and equipment a framework contains guidance on how to. I good design practice for medical devices and equipment a framework by. Good design practice for medical devices 1.
Good design practice for medical devices and equipment a framework by. Karen alexander john clarkson engineering centre university of cambridge and duncan bishop stewart fox cambridge consultants limited. Managing medical devices within a regulatory framework helps administrators designers manufacturers clinical engineers and biomedical support staff to navigate worldwide regulation carefully consider the parameters for medical equipment patient safety anticipate problems with equipment and efficiently manage medical device acquisition budgets throughout the total product life cycle.
Learn best practices for preforming effective efficient design reviews for medical devices.
Impact Of Regulations On Innovation In The Field Of Medical
 Medical Device Design And Development A Definitive Guide
Medical Device Design And Development A Definitive Guide

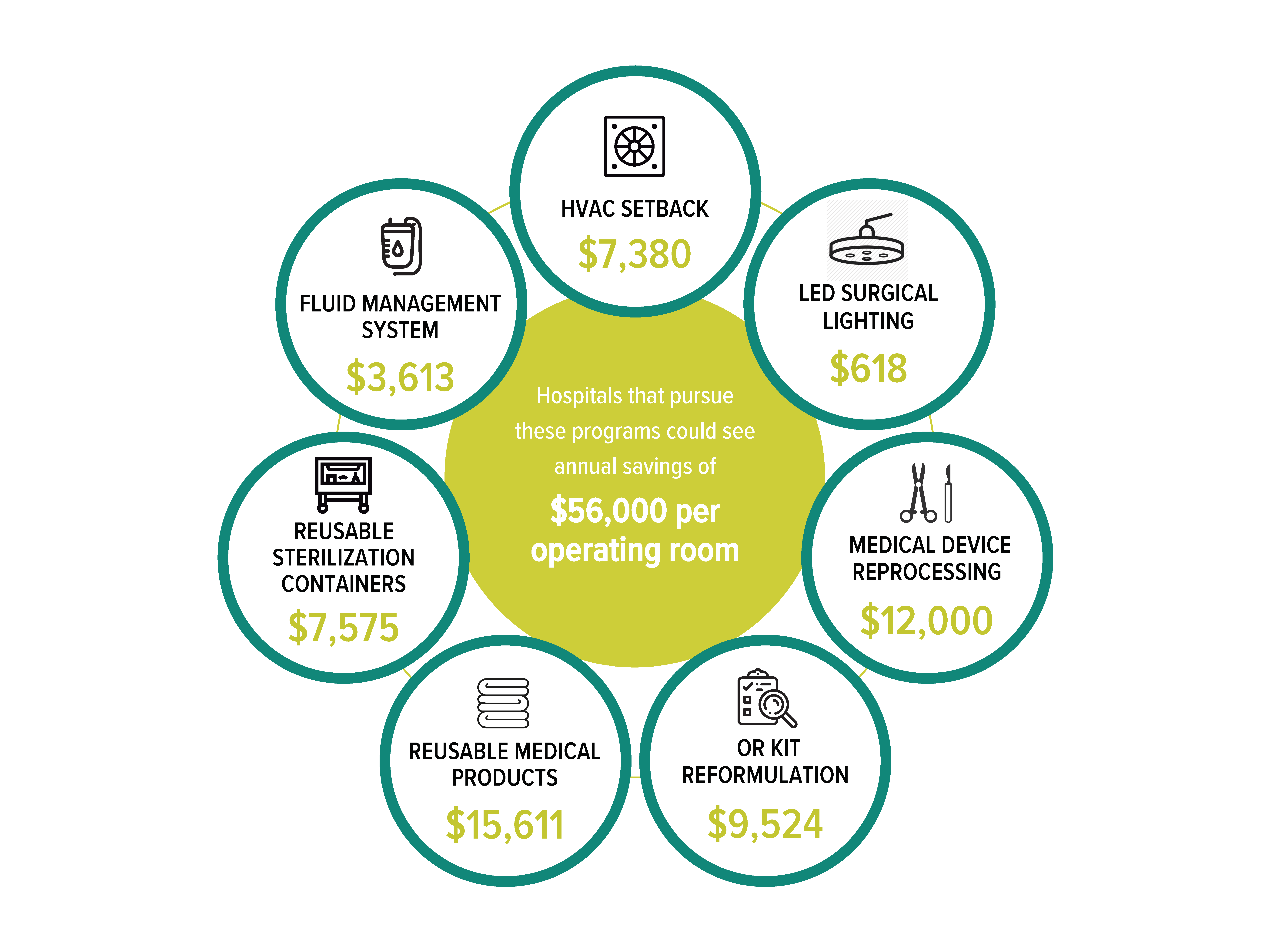 Greening The Or Practice Greenhealth
Greening The Or Practice Greenhealth
Ensuring Privacy And Security In The Healthcare Iot
 Health Canada Regulatory Approval Process For Medical Devices
Health Canada Regulatory Approval Process For Medical Devices
 Medical Device Design Risk Management Basic Principles Wipro
Medical Device Design Risk Management Basic Principles Wipro
 Books
Books
 Iso 13485 Basics And How To Get Started Qms For Medical
Iso 13485 Basics And How To Get Started Qms For Medical
 3d Printing And Fda Regulations For Medical Devices
3d Printing And Fda Regulations For Medical Devices
Ultimate Guide To Medical Device Design And Development Pannam
 Figure 1 From Towards A Framework For Holistic Contextual
Figure 1 From Towards A Framework For Holistic Contextual
 Good Design Practice For Medical Devices
Good Design Practice For Medical Devices
 How To Design And Develop A Mobile Health Application
How To Design And Develop A Mobile Health Application
 Implementing Design Controls For Medical Devices With A
Implementing Design Controls For Medical Devices With A


